Carl Wilhelm Scheele
1742-1786
Scheele was born on December 9th, 1742 in Swedish Pomerania (today Germany). At age of 14, he became an apprentice pharmacist, and got interested in chemistry. In very basic laboratory, Scheele’s outstanding experimental skills enabled him to make many contributions to chemistry, among which the most famous one was the independent discovery of oxygen around 1773. Scheele lived in poverty for most his life. In 1782 he was finally able to setup his own lab, but soon he suffered from poor health and left the world on May 26th, 1786 (aged 43) in Köping, Sweden. His main scientific contributions are:
Discovered oxygen (which Scheele named “fire air”), pointed out that the air is composed of fire air and “foul air” (nitrogen).
Discovered many inorganic substances such as chlorine gas (not knowing it was an element), hydrogen fluoride, and silicon tetrafluoride.
Distinguished graphite and molybdenum disulfide.
Discovered silver salts decomposed under light exposure, which was the basic principle of early photography.
Discovered many organic acids such as tartaric acid and lactic acid.
Scheele's Instruments
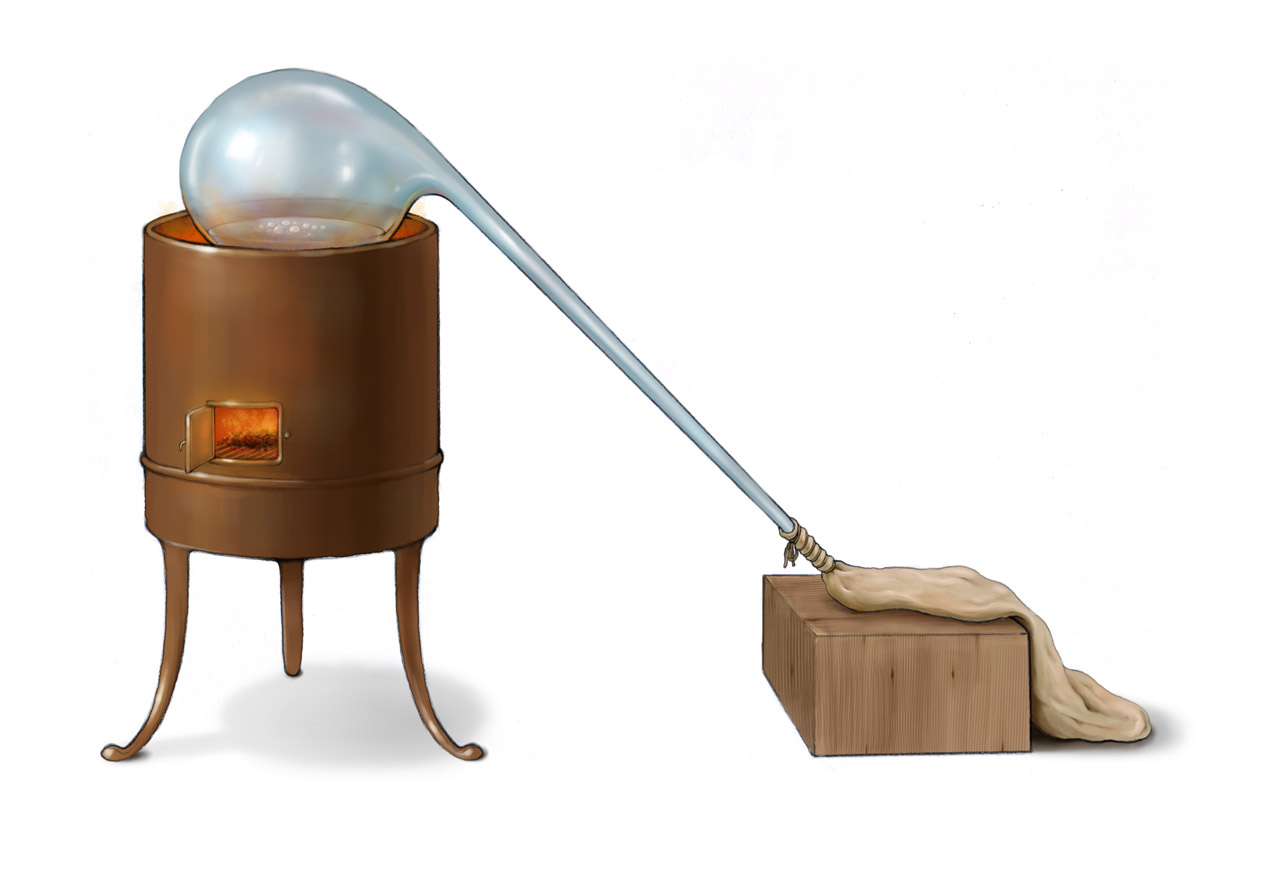
Above is Scheele’s instruments for preparing oxygen described in his Chemische Abhandlung von der Luft und dem Feuer published in 1777 (a photorealistic CG reconstruction can be found here). Inside the retort was a mixture of potassium nitrate and concentrated sulfuric acid. Upon heating the mixture, a colorless gas was released and collected in the bladder tied to the opening of the retort. Scheele observed that combustion became more intense inside this gas, emitting bright light. He named this gas “fire air”, which is the oxygen we know today. He thought that the air around us was composed of fire air which could support combustion and “foul air” (nitrogen) which could not support combustion. Scheele discovered oxygen around 1773. This discovery was published in his book Chemische Abhandlung von der Luft und dem Feuer in 1777, which was later than Priestley’s book. However, it is accepted that two scientists both independently discovered oxygen and should share the honor.
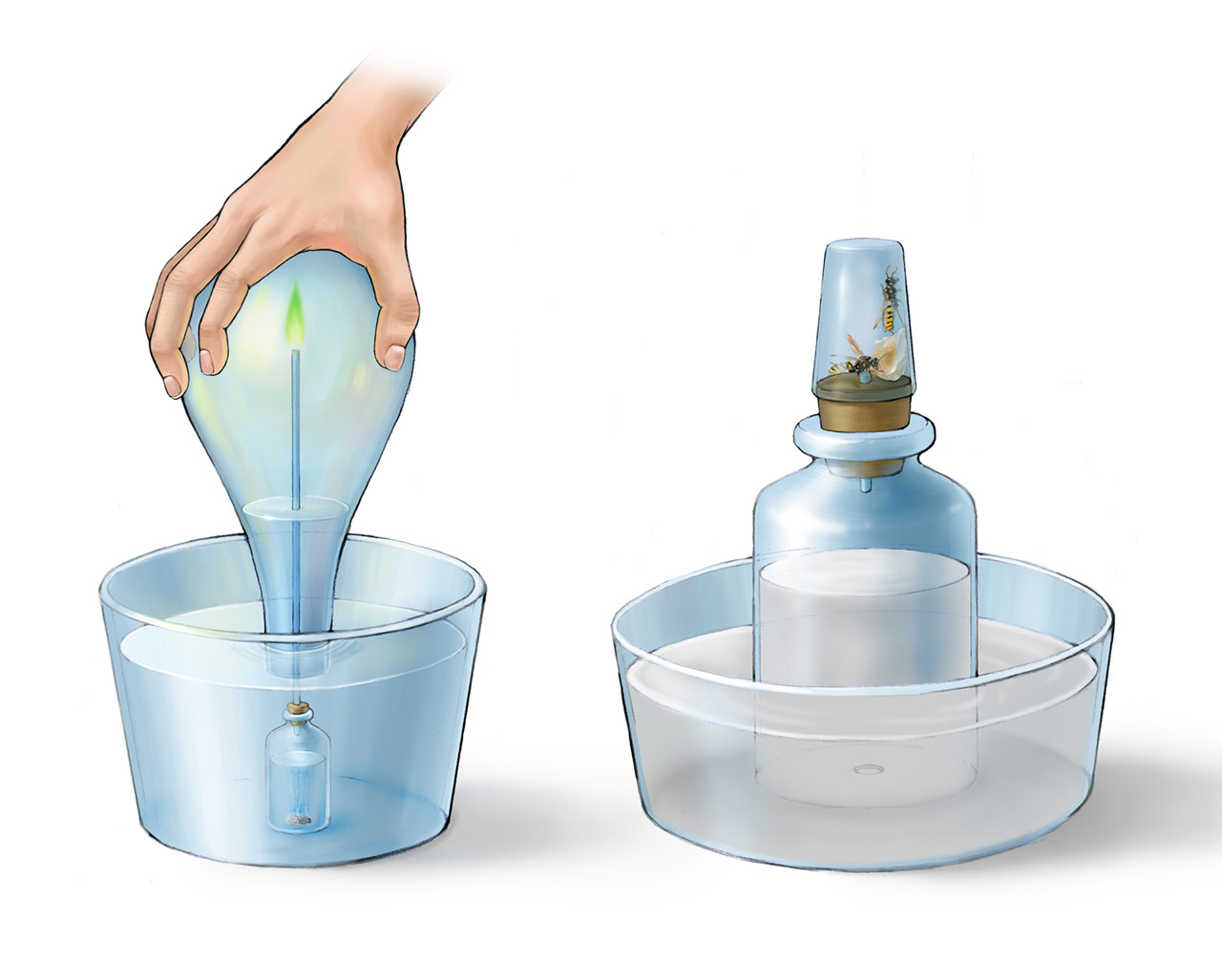
Above is Scheele’s apparatuses for studying hydrogen combustion and animal respiration described in his Chemische Abhandlung von der Luft und dem Feuer published in 1777. Scheele found that burning of hydrogen gave a yellowish green color. Because the combustion consumed the oxygen, the water level inside the inverted container rose up. Scheele used hot water in this experiment, he did not realize that the water condensed on the container was a product of hydrogen combustion. Different from Mayow’s experiment, in Scheele’s experiment of animal respiration, he used both air and oxygen he prepared, and limewater for absorbing carbon dioxide. In addition, the animals he studied were mainly insects. Besides the bees above, he also studied caterpillars and butterflies. For the bees, he carefully prepared some honey inside the container for them to eat. He found a single bee would live twice the time as two bees at the same experimental conditions. When oxygen was used, all the volume inside the container was replaced by limewater at the end of the experiment. Scheele was not a wealthy researcher. He often used ordinary objects for his experiments. For example, the apparatus for studying animal respiration was made of a milk bottle with a hole drilled on the bottom and a common glass cup to hold the bees.
Other Chemists