Justus von Liebig
1803-1873
Liebig was born on May 12th, 1803 in Darmstadt, Grand Duchy of Hesse. He made important contributions to organic chemistry, analytical chemistry, agricultural chemistry, nutritional science, and physiology. In addition, Liebig innovated chemistry education by lecturing and advised his students directly in the labs, and his lab at University of Giessen attracted many students from Germany and other countries. Liebig believed that scientific knowledge should be borderless and he was one of the influential scientists in the 19th century who advocated the internationalization of science. On April 18th, 1873, Liebig died in Munich, Germany at age of 69. His main contributions to science are:
In organic and analytical chemistry, invented new apparatus for chemical elemental analysis, greatly improving its accuracy and efficiency.
In agricultural chemistry, applied organic chemistry in agriculture, pointed out the nutrient sources of chemical elements (such as nitrogen) inside plants, proposed using chemical fertilizer to support plant growth.
In nutritional science and physiology, proposed how nutrients transformed inside bodies from a chemical perspective, correctly predicted that starch and sugar could transform into fat.
Liebig's Instruments
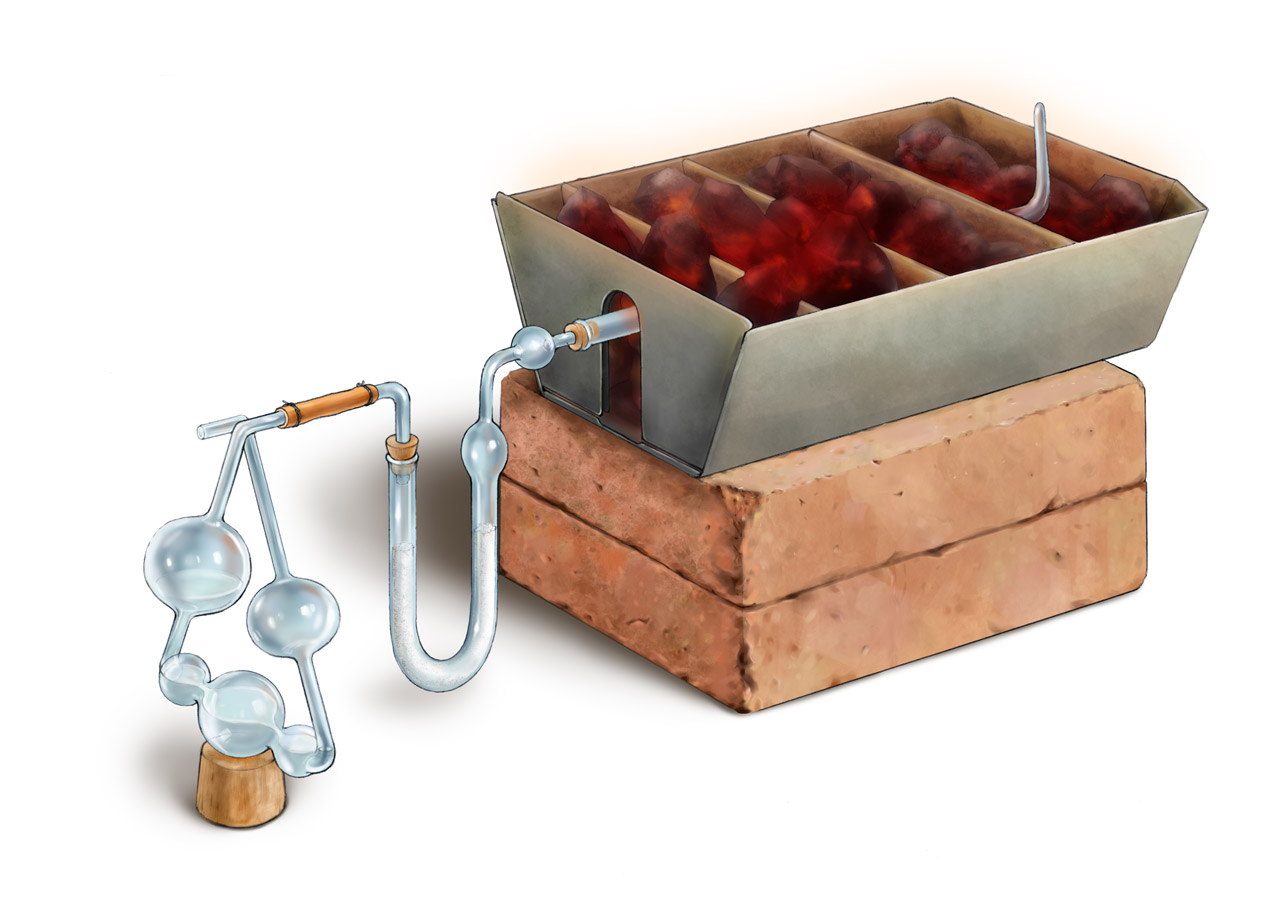
Above is Liebig’s apparatus for organic elemental analysis described in his Anleitung zur Analyse Organischer Körper published in 1837. (The figure was adapted from an online photo of the apparatus in Liebig Museum, a photorealistic CG reconstruction can be found here.) Inside the long tube on the right was the mixture of the organic substance of interest and copper oxide. During the experiment, with heat supplied from burning charcoal in the ion box, copper oxide reacted with the organic substance and produced carbon dioxide and water. Water was absorbed by calcium chloride in the U-shaped tube, and carbon dioxide was absorbed by the potassium hydroxide solution inside the 5-ball apparatus called “kaliapparat”. Its function and the change of water level inside during the experiment was explained below. From the weight of water and carbon dioxide, the amount of hydrogen and carbon could be determined. And the difference between the original weight of the organic substance and the total weight of hydrogen and carbon was the weight of oxygen. (For organic substance with elements other than carbon, oxygen, and hydrogen, such as those with nitrogen, this apparatus was not useful.) Compared to other apparatuses from the same time, Liebig’s apparatus was compact, easy to use, and accurate. As a result, it was quickly spread to many labs all over the world. In addition, the “kaliapparat” can still be found in the logo of American Chemical Society, indicating the significance of this apparatus.
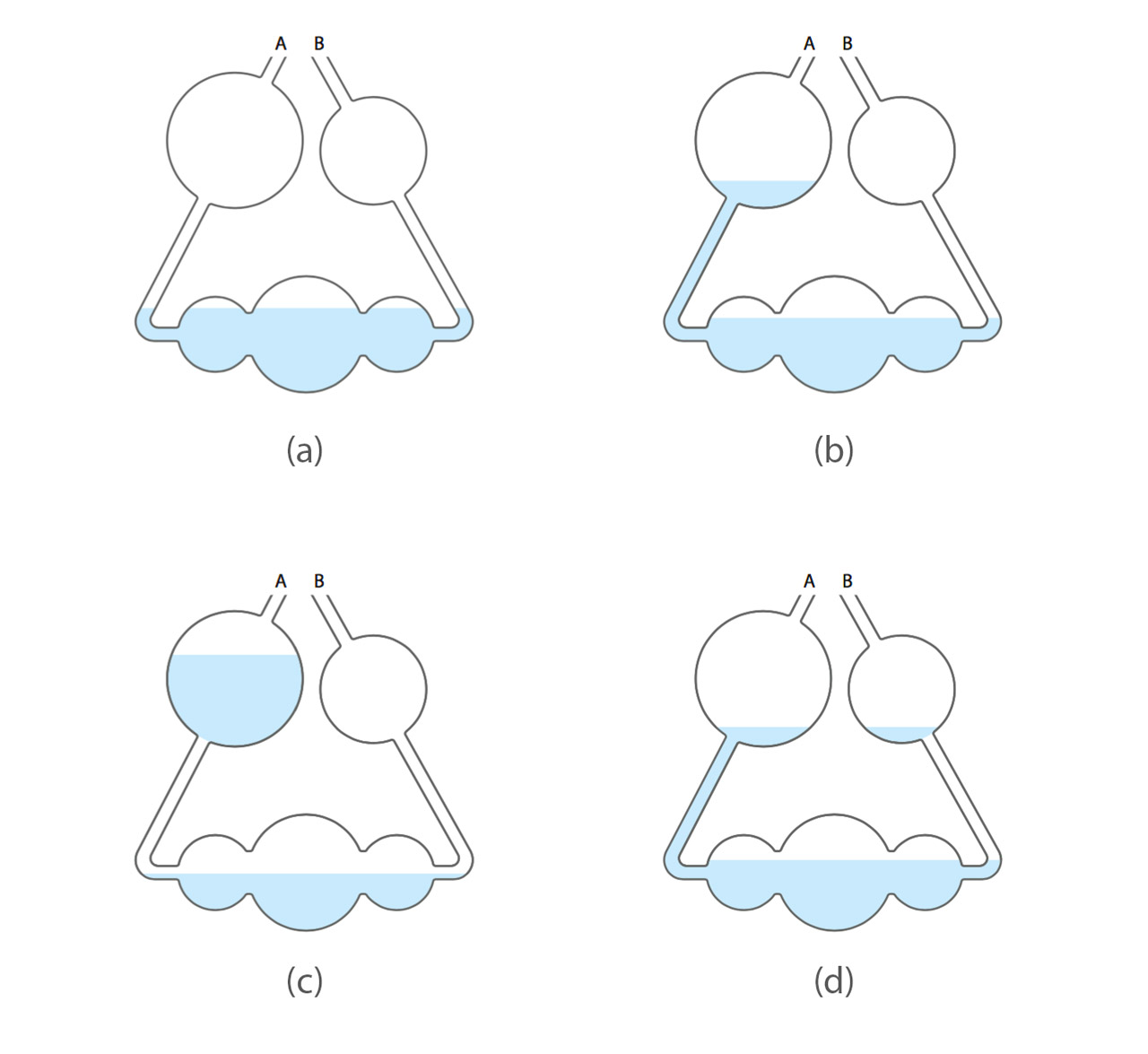
Above: possible liquid levels inside a kaliapparat. (a) Water level after potassium hydroxide solution was added to the kaliapparat. (b) With all devices connected, sucking air from B resulted in the water level raised to the biggest sphere on the left. If the water level did not change over a period of time, the system was airtight. (c) As experiment started, absorption of water and carbon dioxide led to the decrease of pressure inside, resulting in the increase of water level inside the biggest sphere. (d) At the end of experiment, air was introduced into the system by breaking the sharp end of the test tube containing organic substances. The water level inside the biggest sphere dropped and a portion of liquid entered the smaller sphere on the right side.
Other Chemists