Humphry Davy
1778-1829
Davy was born on December 17th, 1778 in Cornwall, England. As a young research, Davy became famous for his research on nitrous oxide, also known as the laughing gas. After Volta invented Voltaic piles, Davy focused his research on electrochemistry, and made important discoveries in this field. Through electrolysis reactions, he discovered many alkali and alkaline earth metals. The safety lamps invented by Davy saved the mining industry in England. In addition, Davy was also a poet. Davy died on May 29th, 1829 (aged 50) in Geneva, Switzerland. His main scientific contributions are:
Discovered the physiological effect of nitrous oxide (laughing gas), demonstrated its effect in public and got the audience interested in science.
Discovered many chemical elements through electrolysis, including alkali metals potassium and sodium, alkaline metals magnesium, calcium, and barium.
Pointed out that chlorine was an element.
Invented safety lamps.
Davy's Instruments
Above is Davy’s apparatus for studying the electrolysis of water, described in his paper On Some Chemical Agencies of Electricity published in 1807. The electrolysis reaction of water was first discovered by two Dutch scientists in 1789, with the electricity generated with an electrostatic machine. After the invention of Voltaic pile, water electrolysis experiments were commonplace. The apparatus above was designed by Davy for study the mechanism of water electrolysis. To avoid contamination from glass container, he used cone-shaped cups made of pure gold. After filling the two cups with distilled water, he used a moistened piece of amianthus to connect to two cups. Ten minutes after the electrolysis started, Davy observed that the water inside cup connected to the positive electrode of a battery could turned litmus paper red, indicating the production of acid. On the other hand, the water inside the cup connected to the negative electrode turned litmus paper blue, indicating the production of alkali.
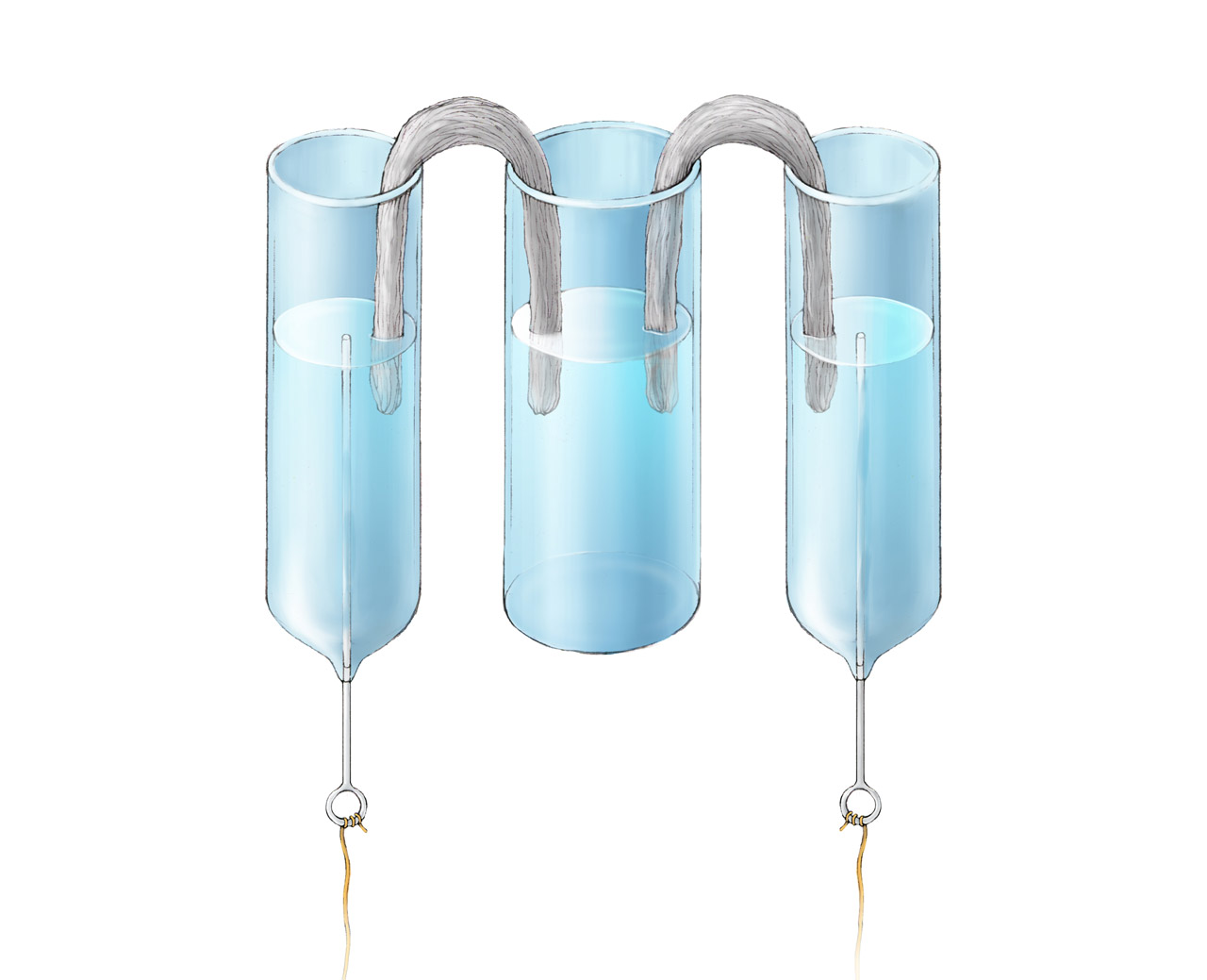
Above was Davy’s apparatus for studying the mechanism of electrolysis, described in his paper On Some Chemical Agencies of Electricity published in 1807. The left cup contained potassium sulfate solution and was connected to the negative electrode of a battery. The middle cup contained distilled water with litmus. The right cup contained distilled water and connected to the positive electrode. Two moistened pieces of amianthus connected three cups. After the electrolysis started, the water around the right amianthus in the middle cup turned red first, which was opposite to Davy’s expectation. Davy thought the acid should be sulfuric acid and came from the left cup with potassium sulfate, hence the water around the left amianthus in the middle cup should turn red first. We now know that positive hydrogen ions were generated near the right negative electrode, they diffused through the right amianthus and turned the water round it in the middle cup red. Although Davy could not give a correct explanation to these results at that time, his work pointed the electrochemistry research in the right direction.
Above is Davy’s safety lamp described in his On the Safety Lamp for Coal Miners, with Some Researchers on Flame in 1818 (a photorealistic CG reconstruction can be found here). The most important part of this safety lamp is the metal wire mesh shielding the flame. Davy discovered that if the flame reached the metal mesh, the heat of the frame would be dissipated quickly through the mesh and its temperature quickly dropped to the point that it was unable to ignite the explosive gas in the environment. Davy’s safety lamp saved the accident-prone coal mine industry in England. Some friends suggest Davy to apply for patents of his invention, which would bring him a fortune. Davy wrote back to his friends: “My good friend, I never thought of such a thing: my sole object was to serve the cause of humanity; and if I have succeeded, I am amply rewarded in the gratifying reflection of having done so… More wealth could not increase either my fame or my happiness. It might undoubtedly enable me to put four horses to my carriage, but what would it avail me to have it said that Sir Humphry drives his carriage and four? ”
Other Chemists