Michael Faraday
1791-1867
Faraday was born on September 22th, 1791 in Newington Butts, England. Despite his humble origins and little formal education at young age, Faraday eventually became one of the most famous scientists in the 19th century. He made great achievements in electromagnetic science and electrochemistry. The electrical generators and motors we used today all thank to Faraday’s discoveries in electromagnetic science. Faraday was also a great science communicator and popularizer. His Christmas lectures in Royal Institution in London were very successful and loved by young people. On August 25th, 1867, Faraday died in Middlesex, England at age of 75. His main scientific contributions are:
In electromagnetism, discovered electromagnetic induction, which was the foundation of electrical generators.
In electrochemistry, discovered Faraday’s laws of electrolysis, making electrochemistry a quantitative science.
In chemistry, liquefied gas (chlorine) for the first time, and discovered new substances such as benzene and hexachloroethane.
In nanoscience, synthesized gold nanoparticles, and discovered they exhibited different properties from bulk gold.
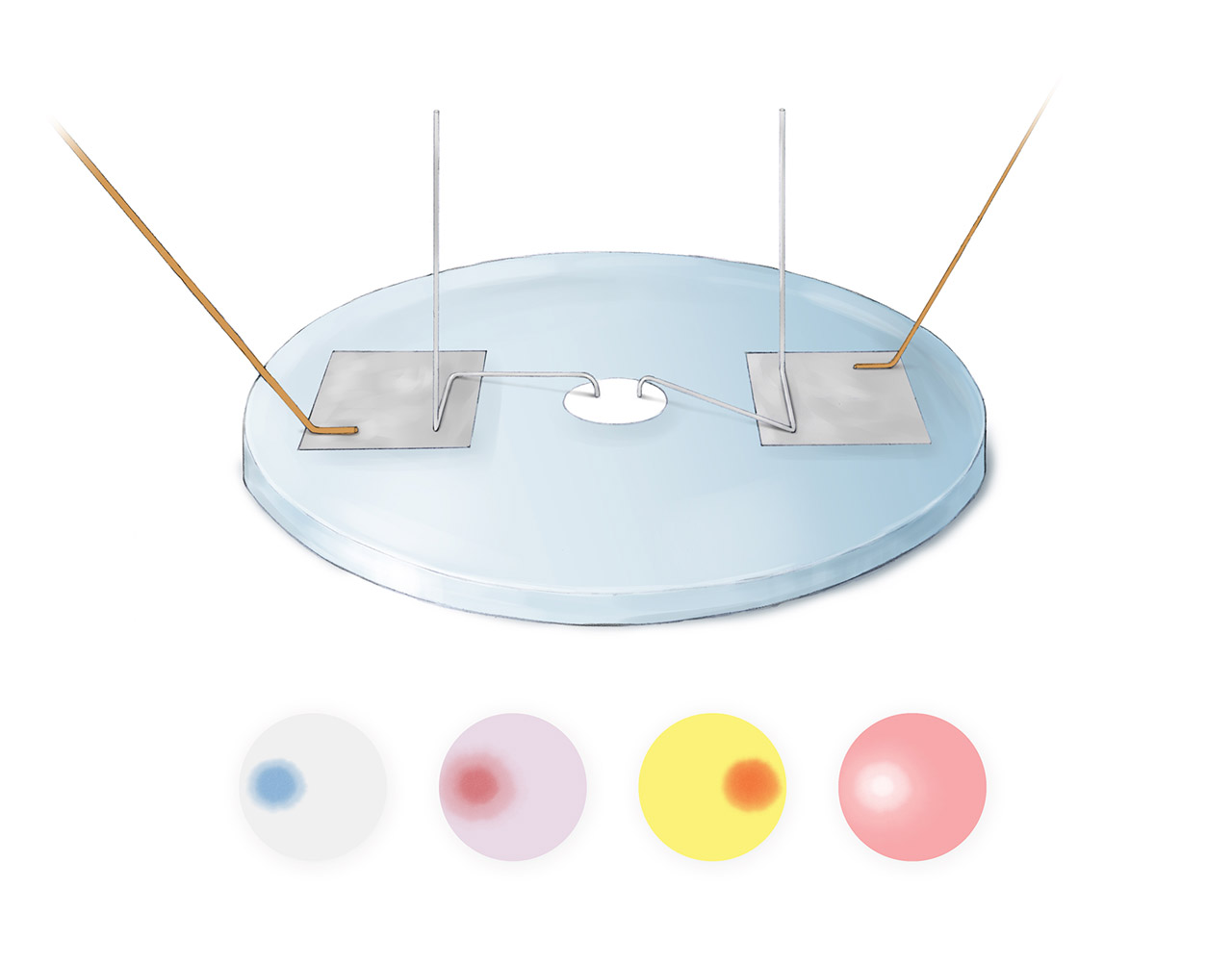
Faraday's Instruments
Above is Faraday’s apparatus for study electrolysis, described in his Experimental Researchers in Electricity published in 1839. Compared to Davy’s apparatus, Faraday’s was simpler and more efficient. On the circular glass plate were two square tin foils and a circular filter paper soaked with a certain chemical solution. The left and right tin foils were connected to the positive and negative electrodes of a battery. Two platinum wires with special shapes and enough weight connected the two foils with the filter paper, completing the circuit. The electrolysis occurred at the locations where the platinum wires touched the filter paper. With this setup, different reactions could be studied simply by switching the filter paper soaked with other chemical solutions. Four results are shown above. For left to right: (1) for paper soaked in a solution with potassium iodide and starch, a blue spot appeared under the positive electrode due to the production of iodine. (2) for paper soaked in a solution with sodium chloride and litmus, a red spot appeared under the positive electrode due to the production of acid. (3) for paper soaked in a solution with sodium sulfate and turmeric, a red spot appeared under the negative electrode due to the production of alkali. (4) for paper soaked in a red solution with hydrochloric acid and litmus, a white spot appeared under the positive electrode due to the production of chlorine that bleached the red color. Based on many experiments, Faraday discovered that the amount of products generated in electrolysis was proportional to the amount of charge passed though the circuit, which is the first law of Faraday’s laws of electrolysis.
Above are Faraday’s apparatuses for measuring the amount of electrical charge during electrolysis, described in this paper On Electrical Decomposition published in 1834. In a series circuit, the amount of charge passing through each of the components is the same. If an apparatus for water electrolysis is connected in series with an electrolysis apparatus of interest, the amount of charge passed through this apparatus can be calculated through measuring the volume of oxygen or hydrogen generated in the water electrolysis. Here we show 3 apparatuses, which were called volta-elecrometer by Faraday. One apparatus separately measured the volumes of hydrogen and oxygen, one measured the volume of either hydrogen or oxygen, the third one measured the total volume of hydrogen and oxygen. Faraday found that mixture of hydrogen and oxygen should not be in contact with platinum electrodes, as they would be converted back to water and affect the accuracy of the experiment. With volta-elecrometers, Faraday was able to determine the accurate atomic weight of many elements and also discovered the second law of electrolysis.
Other Chemists