Polymers
The Establishment of Macromolecular Concept
“Polymer” was initially proposed by “Berzelius” in 1833. However, the concept of “polymer” was totally different at that time. Berzelius used “polymers” to distinguish two types of substances, isomers with the same molecular weight and compounds with the same chemical composition but different molecular weights, for instance, ethylene (C2H4), butylene (C4H8), and hexene (C6H12). According to his definition, both butylene and hexene are polymers of ethylene.
The modern concept of polymer that polymer is macromolecules with giant molecular weights and made up of repeating units of small molecules via covalent bonds is proposed by Hermann Staudinger in 1920. However, most chemists did not believe the existence of macromolecules. Therefore, the concept of macromolecule led to academic debate between scholars that backed small molecules and those that backed macromolecules. Heinrich Wieland, a Nobel Laureate in chemistry, wrote to Staudinger, “Dear colleague, drop the idea of large molecules; organic molecules with a molecular weight higher than 5000 do not exist. Purify your products, such as rubber, then they will crystallize and prove to be low molecular compounds!” The debate lasted for 15 years, but it finally ended up with a thorough victory of the side that favored macromolecule. Afterwards, Werner Kuhn was one of the first to propose a flexible polymer model and used statistical mechanics methods to study polymer chemistry and polymer physics.
The establishment of macromolecular concept greatly prompts the development of polymer materials. Today, the artificially synthesized polymers have spread out to every aspect of our life. For instance, the paint of tables, computer keyboards, the insulating layer of electrical wires, tapes, the fabric of a backpack, the sole of shoes, food bags, beverage bottles, and the cushion on chairs. It’s hard to imagine what our life will be without polymers.
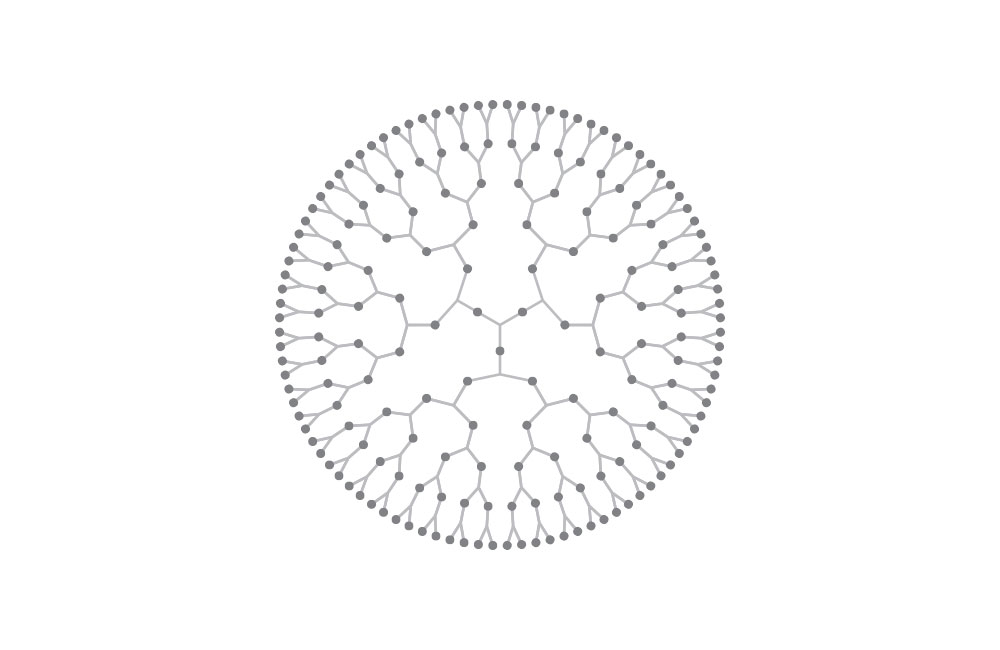
Top diagram: dendritic polymer. Most polymers are linear structure, but some can be dendritic or form 3D networks.
Top: early proposed structure of natural rubber made of small molecules.
Bottom: polymer structure of natural rubber.
Small molecules and macromolecules. The natural rubber is the earliest polymer used by human. C. M. de la Condamine introduced rubber to Europe from South America in 1736. Many polymers were synthesized by chemists in lab. In 1910, the first polymer based plastics, phenolic resin, was commercialized by General Bakelite. However, being in front of many experimental achievements, chemists generally consider polymers were some “assembly” of small molecules through non-covalent interactions (green dash lines in the above image). H. Staudinger proposed the concept of “macromolecule” in 1920-1922 by pointing out that polymers were actually long-chain macromolecules with giant molecular weights and composed of repeating units of small molecules. Natural rubber, starch, and cellulose are all polymers. Staudinger’s macromolecular concept triggered rapid development of polymer science and resulted in a series of important polymers, such as Nylon, polyethylene, and polystyrene that quickly changed our life. Staudinger was awarded Noble Prize in chemistry due to his great contribution in polymer chemistry. [Figure reference: Mülhaupt, R. Angew. Chem. Int. Ed. 43, 1054 (2004)]
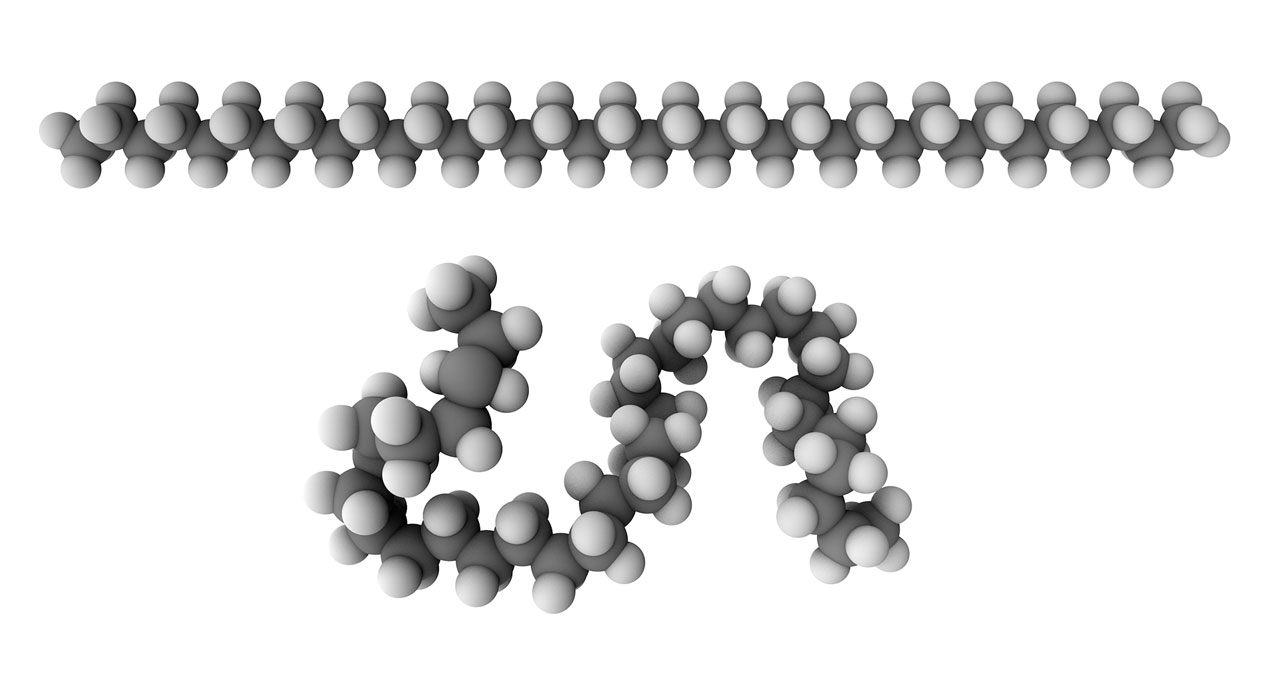
Top: rigid model of polyethylene.
Bottom: Flexible model of polyethylene.
Rigid and flexible molecular chains. Even though Staudinger successfully pointed out the existance of polymer, his polymer model was still defective. Staudinger thought that polymers should regarded as rigid chains and behaved like wooden rods. But it was limited to explain the physical and chemical properties of polymer with his theory. In 1930-1934, Kuhn et al. proposed a flexible-chain model, and firstly applied statistical mechanics and random walk model to study properties such as viscosity. By his efforts, a theory of polymer that more agreed with the experimental observation was developed. Staudinger still insisted on his rigid chain model until 1951, but he gave a positive opinion on Kuhn’s theory in his Noble Prize Speech in 1953. [The figure of rigid and flexible polyethylene model was generated by Chem3D]
Conductive polymers. In 1977, A. Heeger, McDiarmid, and Hideki Shirakawa independently discovered that the electrical conductivity of the polyacetylene film would be increased by several orders of magnitudes when it was treated with iodine vapor. This generated great interests in conductive polymers, because people generally believed that polymers were excellent insulators. Chemists synthesized a series of conductive polymers (above image) after 1977, and the applications of these polymers in light-emitting diodes, transistors, and solar cells marked the emergence of a new interdisciplinary field, plastic electronics. Those three scientists shared the Nobel Prize in chemistry in 2000 due to the discovery of conductive polymers. In the above image, hexagons and pentagons represent benzene, and thiophene, respectively, while red, blue, and orange dots are oxygen, nitrogen, and sulfur, respectively.
Other Topics